20 March 2024
Anatomic Implants, a young medical technology company from Washington, DC, is using additive manufacturing to break new ground in the production of implants for toe joints. Founded in 2016, the company claims to be the first medical technology start-up to have developed and patented a replacement that additively reproduces the first metatarsophalangeal (MTP) joint almost perfectly from titanium.
MTP joints connect the metatarsal bones to the five toe bones. The joint at the base of the big toe is also one of three important points for maintaining balance. It is often the first joint of the foot to develop osteoarthritis.
To bring its product to market, Anatomic Implants is working with the French AM system manufacturer Addup, which is using a FormUp 350 PBF machine to prepare the implant for submission to the FDA.
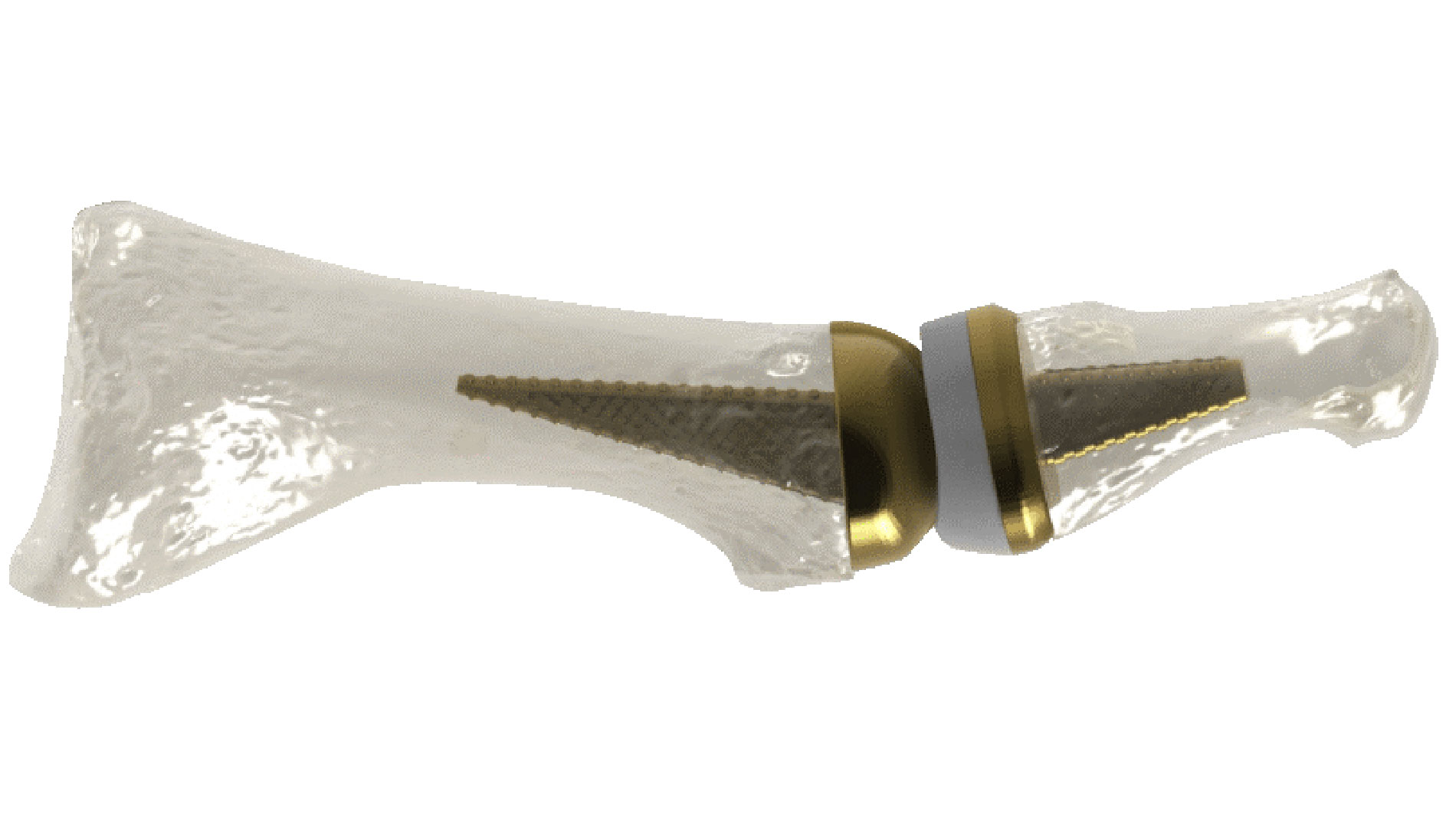
According to Addup, the global market for reconstructing the first MTP joint is worth more than US$500 million per year. However, the very few products available are leaving that market undersupplied. Addup also points out that the existing products are not anatomically compatible and do not have the potential to support bone growth or the anatomy of the big toe joint. Furthermore, additive manufacturing is the only way to integrate a porous structure into the design in order to promote osseointegration. This process gives implants a much greater chance of bonding with bone, which can reduce the likelihood of their being rejected by the body.
“With first MTP joint replacement being a largely underserved market and medical device companies building lattice structures into implantables since the mid 2000s, we sought out to make a more anatomical design by leveraging the latest technologies adopted by the industry and the FDA,” explains David Nutter, president of Anatomic Implants.
The 510(k) clearance process involves a comprehensive review of safety and performance data on an implant to determine if it is substantially equivalent to any implant already on the market. Having already completed several tests, Anatomic Implants is expecting its new product to receive 510(k) clearance in late Q3 2024.
FURTHER INFORMATION:
Tags
- Medical technology
